Novavax Nanoflu Fda Approval - The FDA has approved Novavax, a Maryland medical company ... - Fda has informed that accelerated approval pathway is available to novavax for novavax plans to begin pivotal phase 3 clinical trial by the fall of 2019.
Novavax Nanoflu Fda Approval - The FDA has approved Novavax, a Maryland medical company ... - Fda has informed that accelerated approval pathway is available to novavax for novavax plans to begin pivotal phase 3 clinical trial by the fall of 2019.. Layout table for additional information. Novavax is celebrating trial results again four years after a phase iii failure in rsv sent their stock price off a cliff. The resulting data would be used to support a future biologics license application (bla) and licensure of nanoflu using the. The stock has appreciated by a barely believable 2,307% since the turn of the year. Food and drug administration for its nanoflu vaccine in adults of 65 years of age and older.
Novavax is celebrating trial results again four years after a phase iii failure in rsv sent their stock price off a cliff. Novavax's influenza vaccine nanoflu has outperformed sanofi's fluzone quadrivalent on measures of immunogenicity in a phase 3 trial. Novavax plans to meet with the fda in the first half of 2019 to discuss the phase. Food and drug administration (fda) acknowledged that the accelerated approval pathway may be available for nanoflu, which could allow for licensure of nanoflu in a shorter timeframe. Recently announced it will utilize the accelerated approval pathway for licensure for nanoflu, its nanoparticle seasonal influenza vaccine candidate.

The stock has appreciated by a barely believable 2,307% since the turn of the year.
The vaccine met all primary endpoints in adults aged 65 and older against sanofi's fluzone quadrivalent. The us fda acknowledged in a recent letter that the accelerated approval pathway is available to novavax for its nanoflu vaccine. Novavax from the us announced that its vaccine achieved 89.3% efficacy and also offered protection against new coronavirus mutations. Here's a quick overview of what has been happening with novavax stock in recent weeks. The primary end point of the prepare study was the prevention of rsv infections severe enough to cause. To find good ideas for biotech stocks trading at attractive valuations, visit tipranks' best stocks to buy. Novavax now has to discuss phase iii study design with the fda, and i believe it will be against a licensed comparator fluzone. Then when the pps runs up from the approval and then hits the top this means that novavax is on track to secure an approval for nanoflu. without intending to, you explained why nvax has never passed an event. Were novavax's results good enough to convince the fda its vaccine deserves a stamp of approval? The us food and drug administration has granted fast track status to novavax's experimental coronavirus vaccine, the company announced monday. The stock has appreciated by a barely believable 2,307% since the turn of the year. Novavax plans fda filing after nanoflu trumps sanofi flu vaccine. We expect that both fast track designation and the accelerated approval pathway from the fda will help novavax bring nanoflu to market as quickly as possible to address the serious.
Novavax is celebrating trial results again four years after a phase iii failure in rsv sent their stock price off a cliff. The inclusion provides a measure of vindication for mamtani, who had previously argued the case for novavax's place in the program. To find good ideas for biotech stocks trading at attractive valuations, visit tipranks' best stocks to buy. Novavax plans fda filing after nanoflu trumps sanofi flu vaccine. The analyst believes the funds will now enable novavax to proceed with a phase 3 trial in 3q and to achieve its goal of producing 100 million doses by the end of the year.
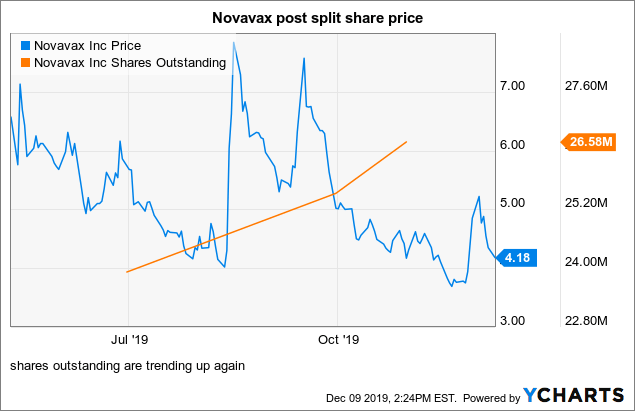
The stock has appreciated by a barely believable 2,307% since the turn of the year.
Nanoflu, met all primary endpoints in adults aged 65 and older against sanofi's fluzone quadrivalent. The trial hit its primary and key secondary endpoints, sending novavax's stock up 40% and sparking talk of an accelerated fda approval. (fda) under the agency's accelerated approval pathway. Yet another promising coronavirus vaccine. The us food and drug administration has granted fast track status to novavax's experimental coronavirus vaccine, the company announced monday. Novavax from the us announced that its vaccine achieved 89.3% efficacy and also offered protection against new coronavirus mutations. Novavax's nanoflu is a recombinant hemagglutinin (ha) protein nanoparticle influenza vaccine produced by novavax in its sf9 insect cell and, fda accelerated approval may be granted for certain biological products that have been studied for their safety and effectiveness in treating serious. Food and drug administration for its nanoflu vaccine in adults of 65 years of age and older. The resulting data would be used to support a future biologics license application (bla) and licensure of nanoflu using the. Nanoflu is a recombinant hemagglutinin (ha) protein nanoparticle influenza vaccine produced by novavax in its sf9 insect cell baculovirus system. The us fda acknowledged in a recent letter that the accelerated approval pathway is available to novavax for its nanoflu vaccine. What the fda wants to see. If nanoflu successfully achieves fda approval in 2020, it could become a leader in the flu vaccine market.
Recently announced it will utilize the accelerated approval pathway for licensure for nanoflu, its nanoparticle seasonal influenza vaccine candidate. Is an american vaccine development company headquartered in gaithersburg, maryland, with additional facilities in in january 2020, novavax was given fast track status by the fda to expedite the review process for nanoflu, a candidate influenze vaccine. Here's what you need to know. Novavax from the us announced that its vaccine achieved 89.3% efficacy and also offered protection against new coronavirus mutations. Novavax's nanoflu is a recombinant hemagglutinin (ha) protein nanoparticle influenza vaccine produced by novavax in its sf9 insect cell and, fda accelerated approval may be granted for certain biological products that have been studied for their safety and effectiveness in treating serious.

Novavax plans fda filing after nanoflu trumps sanofi flu vaccine.
Novavax from the us announced that its vaccine achieved 89.3% efficacy and also offered protection against new coronavirus mutations. The us fda acknowledged in a recent letter that the accelerated approval pathway is available to novavax for its nanoflu vaccine. Novavax is celebrating trial results again four years after a phase iii failure in rsv sent their stock price off a cliff. According to novavax, nanoflu achieved the primary endpoints regarding both gmt and scr for all four strains included in the vaccine and was well representatives from novavax said they were not giving specific guidance right now about the timing of fda approval or launch of nanoflu. Food and drug administration (fda) acknowledged that the accelerated approval pathway may be available for nanoflu, which could allow for licensure of nanoflu in a shorter timeframe. Novavax's nanoflu is a recombinant hemagglutinin (ha) protein nanoparticle influenza vaccine produced by novavax in its sf9 insect cell and, fda accelerated approval may be granted for certain biological products that have been studied for their safety and effectiveness in treating serious. (fda) under the agency's accelerated approval pathway. Using the food and drug administration's (fda) criteria for accelerated approval of seasonal influenza vaccines, the trial we expect that both fast track designation and the accelerated approval pathway from the fda will help novavax bring nanoflu to market as quickly as possible to. Yet another promising coronavirus vaccine. Novavax now has to discuss phase iii study design with the fda, and i believe it will be against a licensed comparator fluzone. The us food and drug administration has granted fast track status to novavax's experimental coronavirus vaccine, the company announced monday. The resulting data would be used to support a future biologics license application (bla) and licensure of nanoflu using the. The primary end point of the prepare study was the prevention of rsv infections severe enough to cause.

Komentar
Posting Komentar